Eve Diagnostics
Eve Diagnostics is the health and wellness arm of Eve Technologies and our key strength lies in providing the most comprehensive and regulated biomarker panel available in the world. Eve Diagnostics is one of only a few laboratories in North America that is regulated for cytokine tests and we are the only laboratory that has been granted large-panel regulation under the Clinical Laboratory Improvement Amendments (CLIA).
Eve Technologies is certified by the Centers for Medicare & Medicaid Services (CMS) as a High Complexity International Laboratory under the CLIA, as defined in U.S. Code of Federal Regulations, Title 42, Chapter IV, Part 493, Laboratory Requirements (42CFR493) 2011 (CLIA# 99D2283230).
We offer Laboratory Developed Tests (LDT) under the speciality Diagnostic Immunology, subspeciality General Immunology. With a test categorization of nonwaived and high complexity testing, this increased complexity parallels increased requirements under CLIA. These assays are not subject to U.S. Food and Drug Administration (FDA) clearance or approval as these tests do not appear on the lists of tests in the Federal Register and the platforms used in specimen analysis have not been reviewed by the U.S. FDA as they are classified as 510(k) exempt.
Clinicians: Check with your State Agency to see if additional Licensure/Registration requirements may apply.

Eve Technologies
Eve Technologies is one of the highest-volume cytokine/immune biomarker facilities in the world and stands as a leading independent corporation committed to empowering biomedical and life science researchers, as well as advancing clinical diagnostics at the forefront of healthcare. With a relentless pursuit of excellence and innovation, we have mastered the cutting-edge assay technology of multiplexing laser beads, ensuring unparalleled precision in our services. As a global industry pioneer and with a client-centric approach we are the preferred choice for researchers seeking accurate and reliable analysis of biological samples.
Established in 2004, Eve Technologies was the first in Canada to effectively adopt multiplex technology and we now have decades of experience in leveraging the latest advancements in Luminex technology. Consequently, Eve Technologies is a resource used widely to meet the testing demands of the world’s leading researchers, serving academia, government agencies, and private sector entities, including pharmaceutical and in-vitro diagnostic companies, and has been instrumental in thousands of published papers in some of the most prestigious journals.
Our impact spans a wide range of research areas, from non-communicable diseases such as hypertension and diabetes to conditions such as Alzheimer’s, irritable bowel, and kidney disease. We have also played a crucial role in understanding immune function in infectious diseases like COVID-19. With hundreds of multiplexing biomarkers in our analyte menu, including categories such as immune response, cardiovascular disease, neuroscience and oncology amongst others, we offer a comprehensive suite of options to suit diverse research needs.
Whilst CLIA certification is only required for human specimens for clinical testing from laboratories in the United States or one of its territories, all analyses performed by Eve Technologies are conducted by the same specialist team within the same established Quality Management System, whether for health science research or Clinical Diagnostics (LDT).

Establishment and verification of assay performance specifications
Eve Diagnostics has established and verified manufacturers’ test system performance specifications for our Clinical Diagnostics (LDT) assays to meet the requirements of CLIA. The establishment and verification of performance specifications provides evidence that the accuracy, precision, analytical sensitivity, and analytical specificity of the procedure satisfies diagnostic adequacy standards as determined by our Laboratory Director.
Performance specifications include:
- Accuracy (the degree to which the measured concentration corresponds to the true concentration of a substance).
- Precision (the closeness of agreement among a series of measurements obtained from multiple sampling of the same homogenous sample).
- Analytical sensitivity (the smallest concentration of a measurand that can be reliably measured by an analytical procedure, or distinguished from a blank) or Lower Limit of Quantification (LLOQ).
- Analytical specificity, to include interfering substances, documented from product information and literature, and general analyte-independent interferences, for example, specimen hemolysis, anticoagulant and lipemia.
- Upper Limit of Quantification (ULOQ).
Reference intervals/normal values encompassing the central 95% of the reference population, as defined by the Clinical and Laboratory Standards Institute (CLSI).
Reportable range of test results for the test system (defined by the LLOQ and the ULOQ of the test system).
Continued reverification of assay performance characteristics to assess graded reactivity.
Our research
Eve Diagnostics is steadfast in its commitment to advancing knowledge and expertise in healthcare science and Clinical Diagnostics. Our ongoing research endeavors aim to enhance our comprehension of the immune environments associated with clinical manifestations in patients with inflammatory, autoimmune, and neoplastic diseases. This, in turn, facilitates more accurate diagnoses and the identification of evidence-based treatment targets. In our publication (Polley et al. Front Immunol 2023), we detail common patterns of immune activation in co-expressing cytokines and their preliminary associations with specific diseases and disease categories.
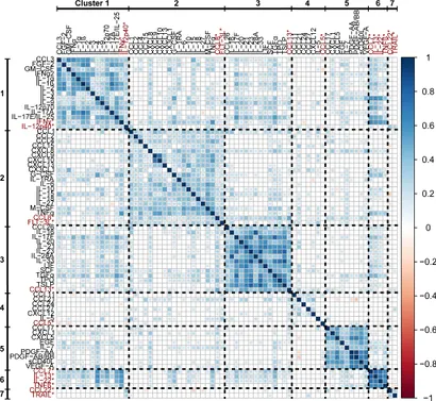
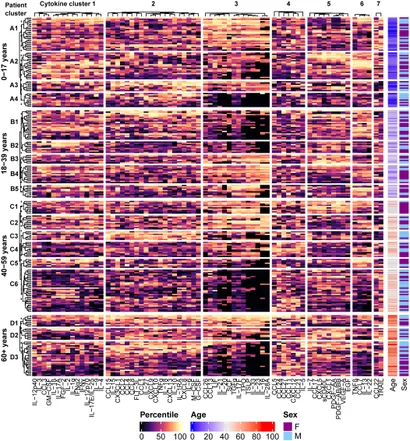
Eve Diagnostics now offers an even larger, expanded, Comprehensive Cytokine, Chemokine, Growth-Factor 95-Plex Panel. This panel builds on the methodology of our previous research and includes updated cytokine clustering and immune signatures to further enhance the interpretation of results.
For detailed results interpretations, click here.

